All Images
Research News
First principles approach to creating new materials

A Perovskite heterostructure. The laws governing the physics of materials are in fact relatively simple, it is just that the behavior of the constituents as a whole are complex. Indeed, materials are made up of atoms whose type, number and arrangement--the crystalline motif--creates distinct properties that emerge through the collective behavior of the seemingly simpler, well-understood parts.
The discovery of emergent phenomena in condensed matter systems is therefore intimately linked with that of discovering the crystalline materials that display these phenomena. Such discoveries by their very nature often occur serendipitously. One challenge is that there are an enormous number of possible bulk compounds that have yet to be identified. As the propensity for new properties tends to increase as the structural and chemical complexity of the crystalline motif increases, the traditional exploratory route to discover these materials is quite demanding. Indeed, new approaches to the discovery of materials displaying novel properties is critical for the continued scientific progress across many disciplines, particularly in the condensed matter sciences, as discussed in detail by the National Academy of Science report "Frontiers in Crystalline Matter: From Discovery to Technology."
Compounding these challenges are recent advances in thin film synthesis techniques that allow for the artificial hetero-structuring of complex materials at the atomic scale (shown here), and for the epitaxial stabilization of single-crystal polymorphs that do not exist in bulk phase diagrams of known compounds, greatly extending the design variables and pushing the rational design of complex oxide materials to a limit where chemical intuition often breaks down.
Thus, it is critical to have a way to understand the intrinsic atomic-scale, structure-property relationships of materials that may or may not yet exist in nature as a means to build our chemical intuition as the initial step in identifying the most promising materials for the painstaking process of laboratory synthesis and characterization.
Credit: Craig Fennie, Cornell University
Download the high-resolution JPG version of the image. (282.6 KB)
Use your mouse to right-click (Mac users may need to Ctrl-click) the link above and choose the option that will save the file or target to your computer.

Craig Fennie, an NSF CAREER awardee, received a MacArthur Fellowship in 2013.
Credit: MacArthur Foundation
Download the high-resolution JPG version of the image. (1.7 MB)
Use your mouse to right-click (Mac users may need to Ctrl-click) the link above and choose the option that will save the file or target to your computer.
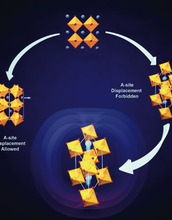
In the search for new classes of multifunctional materials, ferroelectrics, in which the spontaneous electrical polarization couples strongly to other structural, magnetic, orbital and electronic degrees of freedom, is a challenge being actively pursued as a means to achieve electric field-controllable emergent phenomena such as ferromagnetism.
Although perovskites are often what comes to mind when discussing oxide ferroelectricity, the overwhelming majority of oxide perovskite--particularly those which have active electronic, magnetic and orbital microscopic degrees of freedom--adopt highly distorted, non-polar, ground state structures in which the BO6 octahedra are rotated about one or more of the crystal axes. Octahedral rotations, which significantly change the transition metal-oxygen-transition metal bond angle, are well known to control the emergent properties of a given complex oxide material.
A fascinating question that is only recently been considered in earnest concerns how to directly control these octahedral rotations with an external electric field. Our approach to this challenge is to ask the question, "how can octahedral rotations induce a spontaneous polarization, (i.e., ferroelectricity)?" By themselves, octahedral rotations cannot, but recent work by researchers has demonstrated that they can induce ferroelectricity in combination with certain cation ordering and/or hetero-structuring.
Credit: Created by Professor Nicole A. Benedek, UT-Austin; used with permission by The Journal of Physical Chemistry
Download the high-resolution PNG version of the image. (641.3 KB)
Use your mouse to right-click (Mac users may need to Ctrl-click) the link above and choose the option that will save the file or target to your computer.


