Multimedia Gallery
Understanding the ionic activity of membrane surfaces will aid the development of better fuel cells.
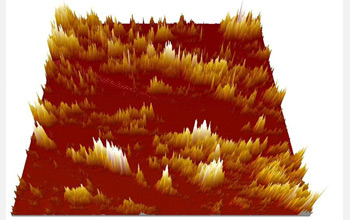
This picture shows that, due to the bi-phasic nature of polymeric proton conducting membranes, only a fraction of a membrane surface is ionic conductive. Consequently, when a catalyst is applied to this surface, only part of the catalyst may be utilized. Such knowledge will be used to develop a membrane with higher surface ionic activity for use in regenerative hydrogen-bromine fuel cell systems, which can efficiently store energy. (This picture is obtained by Electrochemical Atomic Force Microscopy, a technique pioneered by PI Trung Nguyen at the University of Kansas.)
Credit: Trung Nguyen at U. Kansas/ Da-Ming Zhu at U. Missouri - Kansas City
Special Restrictions: For NSF News/Public Information use only.
Images credited to the National Science Foundation, a federal agency, are in the public domain. The images were created by employees of the United States Government as part of their official duties or prepared by contractors as "works for hire" for NSF. You may freely use NSF-credited images and, at your discretion, credit NSF with a "Courtesy: National Science Foundation" notation.
Additional information about general usage can be found in Conditions.
Also Available:
Download the high-resolution JPG version of the image. (63 KB)
Use your mouse to right-click (Mac users may need to Ctrl-click) the link above and choose the option that will save the file or target to your computer.
Related story: Exploring Sustainability for Energy and Buildings