Multimedia Gallery
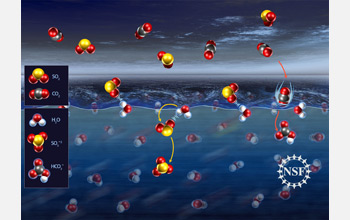
Sulfur dioxide molecules in the atmosphere form a weak bond with water molecules.
Geraldine Richmond and her team at the University of Oregon were surprised to find that molecules of sulfur dioxide (SO2) in the atmosphere tend to form a weak bond with water molecules at the surface of the liquid before finally submerging, whereas carbon dioxide (CO2) molecules dive right in.
"This is the first time that anyone has ever measured, with this level of molecular detail, a gas-surface complex at the surface of liquid water," Richmond said. "We are now investigating a whole series of important environmental gases, ions and solutes at the water surface."
Credit: Nicolle Rager-Fuller
Images credited to the National Science Foundation, a federal agency, are in the public domain. The images were created by employees of the United States Government as part of their official duties or prepared by contractors as "works for hire" for NSF. You may freely use NSF-credited images and, at your discretion, credit NSF with a "Courtesy: National Science Foundation" notation.
Additional information about general usage can be found in Conditions.
Also Available:
Download the high-resolution JPG version of the image. (372 KB)
Use your mouse to right-click (Mac users may need to Ctrl-click) the link above and choose the option that will save the file or target to your computer.
Related story: The Water Dance