All Images
News Release 08-011
Lithium and Beryllium No Longer "Lack Chemistry"
Scientists predict antisocial metals will bond under high-pressure conditions
This material is available primarily for archival purposes. Telephone numbers or other contact information may be out of date; please see current contact information at media contacts.
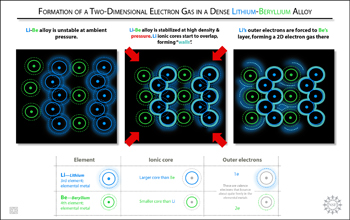
At standard atmospheric or ambient pressure, the lithium beryllium (LiBe) alloy is unstable. However, at high density and at relatively high pressure, the predicted alloy stabilizes. As the atoms are squeezed in tightly, lithium's ionic cores (the larger of the two) begin to overlap. This creates a sort of "wall" that forces the outer (valence) electrons out of the lithium layer, and over to the beryllium layer. It is there that the electrons form a curious two-dimensional gas. In contrast, electrons in most metals bounce about quite freely in a three-dimensional fashion.
Credit: Zina Deretsky, National Science Foundation
Download the high-resolution JPG version of the image. (832 KB)
Use your mouse to right-click (Mac users may need to Ctrl-click) the link above and choose the option that will save the file or target to your computer.

Lithium (Li) and beryllium (Be) form no compounds under normal atmospheric pressure. But under high pressure at least four ordered alloys of these elements are predicted. The bottom left structure is the most unexpected predicted alloy and may have potential for superconductivity.
Credit: Ji Feng, Richard G. Hennig, N.W. Ashcroft, and Roald Hoffmann
Download the high-resolution JPG version of the image. (698 KB)
Use your mouse to right-click (Mac users may need to Ctrl-click) the link above and choose the option that will save the file or target to your computer.


