News Release 04-055
Oldest Hemoglobin Ancestors Offer Clues to Earliest Oxygen-Based Life
Close look at structure of transport proteins could aid search for future blood substitutes
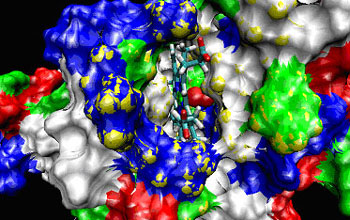
This snapshot comes from an animated simulation of the molecular dynamics of an A. pernix ...
April 20, 2004
This material is available primarily for archival purposes. Telephone numbers or other contact information may be out of date; please see current contact information at media contacts.
ARLINGTON, Va.—Red-blooded genealogists take note: The discovery in microbes of two oxygen-packing proteins, the earliest known ancestors to hemoglobin, brings scientists closer to identifying the earliest life forms to use oxygen.
According to the project's lead investigator, University of Hawaii microbiologist Maqsudul Alam, the research may also aid in the search for blood substitutes as new molecular details shed light on how the structure of such proteins, called protoglobins, evolved to transport and release oxygen.
Scientists from the Maui High Performance Computing Center and the University of Texas Southwestern Medical Center contributed to the research. The findings will appear in the Proceedings of the National Academy of Sciences (PNAS) in an online "Early Edition" this week (at www.pnas.org) and in the April 27 print issue. A four-year, $500,000 grant from the National Science Foundation supported the project.
To life on primordial Earth, oxygen was poison. Within single-celled archaea, special proteins arose that captured and transported molecular oxygen, not to release it for respiration but to isolate and detoxify it to protect the organism. Archaea are a distinct group of microbes. Their lineage diverged long ago from a common ancestor they shared with bacteria and eukaryotes (plants, animals and other life forms that encase their DNA within a nucleus). Many strains of archaea exist, often in the planet's harshest, hottest and oxygen-deprived environments. Some, however, adapted to use oxygen.
Alam's research group found the two primitive protoglobulins in two different archaea species. One, Aeropyrum pernix, is limited to oxygen-based respiration, survives optimally in near-boiling saltwater, and was first discovered among thermal sea vents off Japan. The other, Methanosarcina acetivorans, uses several anaerobic – or oxygen-free – metabolic pathways that create methane gas. M. acetivorans is found in a wide range of realms, including lake-bottom muck, composting leaves, cow pies and human intestines. The genomes of both have recently been sequenced.
The ability to use oxygen for respiration allowed the diversity of life to expand vastly, an impact more fundamental, if perhaps not as dramatic, as the evolutionary transitions organisms made adapting from sea to land, from the ground to the air, or from "all fours" to upright.
Elizabeth Hood, who directs the areas of signal transduction and cellular regulation for NSF's Division of Molecular and Cellular Biosciences, said, "As early life forms were established on earth, the atmosphere contained numerous toxic molecules, including nitric oxide and hydrogen sulfide. Early hemoglobins most likely evolved to bind and detoxify these gases. When oxygen became a component of the atmosphere, it was also toxic, and these early organisms used hemoglobin to bind and ultimately detoxify the oxygen."
However, for advanced and larger life forms to exist in an oxygen-rich atmosphere on land, a mechanism was needed to take advantage of oxygen's benefits, Hood said, and hemoglobins evolved into oxygen carriers rather than detoxifiers.
"Finding early hemoglobins in the most primitive life forms on earth testifies to their crucial role in the development of life as we know it today," she said.
(In humans, with each breath in, hemoglobin binds oxygen in the lungs. Then, carried by blood cells made red by its oxygenated presence, the protein transports oxygen to tissues near and far in the body, where it then releases oxygen, which is essential to cellular respiration.)
To find the two protoglobins, the research team used advanced tools of biotechnology and high-performance computing, cloning genetic sequences from the two microbes and using specialized E. coli bacteria as gene-expression machinery to produce samples of the proteins. To analyze their structures, the team compared alignments with other members of the hemoglobin family of compounds. Computers generated models and created "molecular dynamic simulations" that illustrate with animations how the proteins bind with carbon monoxide, nitric oxide and oxygen.
Genetic sequences, binding characteristics and molecular structures of protoglobins were compared with those of hemoglobins and other oxygen-transport molecules from a wide range of organisms, including bacteria, tubeworms, roundworms, segmented "bloodworms," mice, humans and sperm whales.
According to Alam, the similarities between these molecules and the protoglobins of A. pernix and M. acetivorans suggest "intriguing connections" between them and the evolution of mechanisms that sense oxygen, carbon monoxide, nitric oxide and hydrogen sulfide. These similarities, he said, also suggest connections to LUCA, short-hand for the "Last Universal Common Ancestor."
"LUCA is believed to have been a metabolically 'flexible' single-celled organism with the ability to utilize oxygen for energy before free oxygen even existed in the air," said Alam. "We think protoglobin helped give life to LUCA. And its descendents – hemoglobin, myoglobin, neuroglobin, and cytoglobin – allowed higher organisms to evolve" by allowing organisms to maintain a metabolic balance in an oxygenated world.
-NSF-
Images/B-Roll: An animation simulating the molecular dynamics of an archaeal protoglobin model is available here: http://www.hawaii.edu/microbiology/Alam/globins.htm. A still image from this animation is also available.
NSF's Division of Molecular and Cellular Biosciences: http://www.nsf.gov/bio/mcb/
Alam's Lab website: http://www.hawaii.edu/microbiology/Alam
Hemoglobin, profiled as Protein Data Bank's Molecule of the Month: http://www.rcsb.org/pdb/molecules/pdb41_1.html
Introduction to Archaea (from "The Phylogeny of Life" exhibit at University of California's Museum of Paleontology): http://www.ucmp.berkeley.edu/archaea/archaea.html
Methanosarcina acetivorans profile by Genome News Network: http://www.genomenewsnetwork.org/articles/04_02/m_acetivorans_seq.shtml
Media Contacts
Sean Kearns, NSF, (703) 292-7963, email: skearns@nsf.gov
Program Contacts
Elizabeth E. Hood, NSF, (703) 292-7120, email: ehood@nsf.gov
Principal Investigators
Maqsudul Alam, University of Hawaii, (808) 956-8121, email: alam@hawaii.edu
The U.S. National Science Foundation propels the nation forward by advancing fundamental research in all fields of science and engineering. NSF supports research and people by providing facilities, instruments and funding to support their ingenuity and sustain the U.S. as a global leader in research and innovation. With a fiscal year 2023 budget of $9.5 billion, NSF funds reach all 50 states through grants to nearly 2,000 colleges, universities and institutions. Each year, NSF receives more than 40,000 competitive proposals and makes about 11,000 new awards. Those awards include support for cooperative research with industry, Arctic and Antarctic research and operations, and U.S. participation in international scientific efforts.
Connect with us online
NSF website: nsf.gov
NSF News: nsf.gov/news
For News Media: nsf.gov/news/newsroom
Statistics: nsf.gov/statistics/
Awards database: nsf.gov/awardsearch/
Follow us on social
Twitter: twitter.com/NSF
Facebook: facebook.com/US.NSF
Instagram: instagram.com/nsfgov


