News Release 11-145
Chemists Create Molecular "Flasks"
Researchers design a self-assembling material that can house other molecules

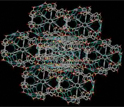
The new material self-assembles into well-defined polyhedra, which organize into a crystal lattice.
July 21, 2011
View an animation that shows a portion of the cubic zeolite-like structure formed by the polyhedra.
This material is available primarily for archival purposes. Telephone numbers or other contact information may be out of date; please see current contact information at media contacts.
Chemical reactions happen all of the time: Some things burn or rust; others react to light exposure. Even batteries use chemical reactions to supply electricity. One of the big challenges chemists continually face is finding new ways to control these reactions or create conditions that promote desirable reactions and limit undesirable ones.
Recently, researchers at New York University (NYU) demonstrated an ability to make new materials with empty space on the inside, an advancement that could potentially control desired and unwanted chemical reactions.
Mike Ward, of NYU's department of chemistry, and a team of researchers created molecular "flasks," which are essentially self-assembling cage-like containers capable of housing other compounds inside them. These flasks may eventually allow researchers to isolate certain chemical reactions within or outside the flask.
The research is published in the July 22, 2011 issue of the journal Science.
"We wanted to create frameworks to serve as the 'hotel' for 'guest' molecules, which can deliver the function independent of framework design," said Ward. "This makes it possible to separate chemicals based on size or perform reactions inside well-defined cages, which could potentially give you more control over chemical reactivity and reaction products. Moreover, these frameworks may prove ideal for encapsulating a wide range of guest molecules, producing materials with new optical or magnetic properties."
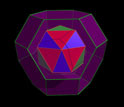
The molecular flasks described by Ward and his collaborators take the shape of a truncated octahedron, one of 13 shapes described as an Archimedean solid, discovered by the Greek mathematician Archimedes. Archimedean solids are characterized by a specific number of sides that meet at corners which are all identical. The regularity of these shapes often means they are of particular interest to chemists and materials researchers looking to create complex materials that assemble themselves.
The extraordinary aspect of this work, supported by the National Science Foundation (NSF), is the self-assembly of the molecular tiles into a polyhedron, a well-defined, three-dimensional, geometric solid. The individual polyhedra assemble themselves using the attractive interactions associated with hydrogen bonds. They then further organize into a crystal lattice that resembles a porous structure called zeolite, an absorbent material with many industrial uses.
The new material differs from zeolite because it is constructed from organic building blocks rather than inorganic ones, which make it more versatile and easier to engineer. In general, inorganic compounds are considered mineral in origin, while organic compounds are considered biological in origin.
This discovery paves the way towards development of a new class of solids with properties that may prove useful for a range of industrial and consumer products.
"By using geometric design principles and very simple chemical precursors, the Ward group has been able to construct relatively sturdy materials which contain many identically sized and shaped cavities," explained Michael Scott, program director in the Division of Materials Research at NSF. "The hollow space inside these materials offers many exciting opportunities for chemists to do things such as isolate unstable molecules, catalyze unknown reactions and separate important chemical compounds."
Future research projects will try to create other types of Archimedean solids or use the truncated octahedron to house different types of functional molecules.
-NSF-
-
View Video
This animation shows a portion of the cubic zeolite-like structure formed by the polyhedra.
Credit and Larger Version -
This image is a representation of a compound (red/blue) nesting inside the new material (purple).
Credit and Larger Version -
The researchers' results are described in the July 22 issue of the journal Science.
Credit and Larger Version
Media Contacts
Lisa Van Pay, NSF, (703) 292-8796, email: lvanpay@nsf.gov
James Devitt, New York University, (212) 998-6808, email: james.devitt@nyu.edu
Program Contacts
Linda S. Sapochak, NSF, (703) 292-4932, email: lsapocha@nsf.gov
Principal Investigators
Michael D. Ward, New York University, (212) 998-8439, email: mdw3@nyu.edu
The U.S. National Science Foundation propels the nation forward by advancing fundamental research in all fields of science and engineering. NSF supports research and people by providing facilities, instruments and funding to support their ingenuity and sustain the U.S. as a global leader in research and innovation. With a fiscal year 2023 budget of $9.5 billion, NSF funds reach all 50 states through grants to nearly 2,000 colleges, universities and institutions. Each year, NSF receives more than 40,000 competitive proposals and makes about 11,000 new awards. Those awards include support for cooperative research with industry, Arctic and Antarctic research and operations, and U.S. participation in international scientific efforts.
Connect with us online
NSF website: nsf.gov
NSF News: nsf.gov/news
For News Media: nsf.gov/news/newsroom
Statistics: nsf.gov/statistics/
Awards database: nsf.gov/awardsearch/
Follow us on social
Twitter: twitter.com/NSF
Facebook: facebook.com/US.NSF
Instagram: instagram.com/nsfgov