We support breakthroughs in chemistry and materials science that enhance nearly every aspect of daily life, from pharmaceuticals to plastics, environmental cleanup to battling pandemics.
NSF-backed research has led to new ways to identify infection gateways of the coronavirus, made an aerogel out of common egg whites that can filter water and store energy, and devised methods to break down or reuse plastic waste.
What we support

Discovery and innovation in chemistry
We support research on the composition, structure, property and reactivity of the basic components of matter.
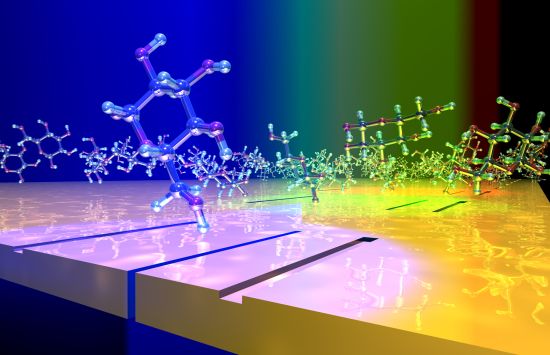
Exploring new materials
We support the study, design and synthesis of materials — from nanomaterials to biomaterials, ceramics to metals.

World-class research centers and facilities
We support a network of research centers and user facilities that tackle grand scientific and societal challenges and train the next generation of chemists and materials researchers. Our programs include: