Multimedia Gallery
Hydrogen Separation Method
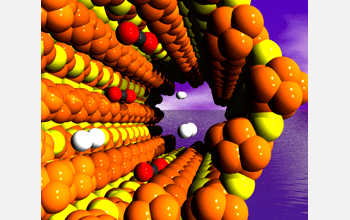
Hydrogen (white) passes quickly through the pores of a new material developed at Northwestern University, while carbon dioxide (red and black) interacts with the walls, slowing it down. The materials, a new family of germanium-rich chalcogenides, offer a new way to separate gases not available before.
More about this Image
Mercouri G. Kanatzidis, a chemist at Northwestern University, together with Gerasimos S. Armatas, a postdoctoral research associate, has developed a class of new porous materials, structured like honey comb, that is very effective at separating hydrogen from complex gas mixtures. The materials are a new family of germanium-rich chalcogenides.
Hydrogen has potential as an alternative energy source, but it has to be purified before it can be used as fuel for fuel cells, and current methods of doing this are not very clean or efficient. The materials developed at Northwestern exhibit the best selectivity in separating hydrogen from carbon dioxide (CO2) and methane, thus far, to the best of the researchers' knowledge. With a more selective process, there are fewer cycles to produce pure hydrogen, increasing efficiency. "Our materials could be used very effectively as membranes for gas separation. We have demonstrated their superior performance," said Kanatzidis.
Currently when hydrogen is produced, the first step yields a mixture of hydrogen and CO2 or hydrogen combined with CO2 and methane. The next step involves removing the hydrogen from these mixtures, but the technology used to do this separates the gas molecules based on their size, which is difficult to do. Instead of size, the materials developed by Kanatzidis and Armatas rely on polarization--the interaction of gas molecules with the walls of the material as the molecules move through the membrane--and is the basis of the new separation method.
Kanatzidis says the soft-walled atoms like to interact with other soft molecules as they pass by, slowing them down as they pass through the membrane. But hydrogen--which is the smallest element--is a "hard" molecule, so it zips right through while softer molecules, like CO2 and methane, take more time.
Kanatzidis and Armatas tested their membrane on a complex mixture of four gases and found that hydrogen passed through first, followed in order by CO2, methane and carbon dioxide. As the smallest and hardest molecule, hydrogen interacted the least with the membrane, and CO2--as the softest molecule of the four--interacted the most.
According to the researchers, small-molecule diffusion through porous materials is a nanoscopic phenomenon. All the pores in the hexagonal honeycomb structure are ordered and parallel, with each hole approximately two to three nanometers wide. All of the gas molecules are at least half a nanometer wide. [Research supported by National Science Foundation grant EEC 06-47560.] (Date of Image: 2008)
SORRY: THIS IMAGE IS NOT AVAILABLE IN HIGH-RESOLUTION FORMAT
Credit: Dr. Gerasimos Armatas and professor Mercouri Kanatzidis
Images and other media in the National Science Foundation Multimedia Gallery are available for use in print and electronic material by NSF employees, members of the media, university staff, teachers and the general public. All media in the gallery are intended for personal, educational and nonprofit/non-commercial use only.
Images credited to the National Science Foundation, a federal agency, are in the public domain. The images were created by employees of the United States Government as part of their official duties or prepared by contractors as "works for hire" for NSF. You may freely use NSF-credited images and, at your discretion, credit NSF with a "Courtesy: National Science Foundation" notation.
Additional information about general usage can be found in Conditions.
Also Available:
Download the high-resolution JPG version of the image. (497 KB)
Use your mouse to right-click (Mac users may need to Ctrl-click) the link above and choose the option that will save the file or target to your computer.