Multimedia Gallery
The search for new classes of multifunctional materials involves many questions.
In the search for new classes of multifunctional materials, ferroelectrics, in which the spontaneous electrical polarization couples strongly to other structural, magnetic, orbital and electronic degrees of freedom, is a challenge being actively pursued as a means to achieve electric field-controllable emergent phenomena such as ferromagnetism.
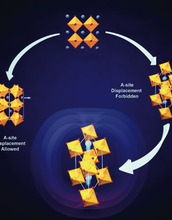
Although perovskites are often what comes to mind when discussing oxide ferroelectricity, the overwhelming majority of oxide perovskite--particularly those which have active electronic, magnetic and orbital microscopic degrees of freedom--adopt highly distorted, non-polar, ground state structures in which the BO6 octahedra are rotated about one or more of the crystal axes. Octahedral rotations, which significantly change the transition metal-oxygen-transition metal bond angle, are well known to control the emergent properties of a given complex oxide material.
A fascinating question that is only recently been considered in earnest concerns how to directly control these octahedral rotations with an external electric field. Our approach to this challenge is to ask the question, "how can octahedral rotations induce a spontaneous polarization, (i.e., ferroelectricity)?" By themselves, octahedral rotations cannot, but recent work by researchers has demonstrated that they can induce ferroelectricity in combination with certain cation ordering and/or hetero-structuring.
Credit: Created by Professor Nicole A. Benedek, UT-Austin; used with permission by The Journal of Physical Chemistry
Images credited to the National Science Foundation, a federal agency, are in the public domain. The images were created by employees of the United States Government as part of their official duties or prepared by contractors as "works for hire" for NSF. You may freely use NSF-credited images and, at your discretion, credit NSF with a "Courtesy: National Science Foundation" notation.
Additional information about general usage can be found in Conditions.
Also Available:
Download the high-resolution PNG version of the image. (641.3 KB)
Use your mouse to right-click (Mac users may need to Ctrl-click) the link above and choose the option that will save the file or target to your computer.
Related story: First principles approach to creating new materials