News Release 04-154
Carbon Nanotubes Yield a New Class of Biological Sensors
Glucose sensor provides real-time readouts without the need to draw blood samples
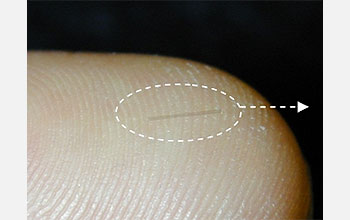
This glass capillary tube has been loaded with glucose-sensitive nanotubes.
December 13, 2004
This material is available primarily for archival purposes. Telephone numbers or other contact information may be out of date; please see current contact information at media contacts.
Arlington, Va.--Nanotechnology researchers at the University of Illinois in Urbana-Champaign have demonstrated a tiny, implantable detector that could one day allow diabetics to monitor their glucose levels continuously—without ever having to draw a blood sample.
The work, which is the first application of a whole new class of biological sensors, was funded by the National Science Foundation (NSF) and announced December 12 in the online edition of the journal Nature Materials.
Principal investigator Michael Strano, a professor of chemical and biomolecular engineering at Illinois, explains that the new sensors are based on single-walled carbon nanotubes: cylindrical molecules whose sides are formed from a lattice of carbon atoms. The idea is to exploit the nanotubes’ ability to fluoresce, or glow, when illuminated by certain wavelengths of infrared light—“a region of the spectrum where human tissue and biological fluids are particularly transparent,” says Strano.
To make a sensor, Strano and his collaborators first coat the nanotubes with a “molecular sheath”: a one-molecule-thick layer of compounds that react strongly with a particular chemical—in this case, glucose. The mix of compounds is chosen so that the reaction also changes the nanotubes’ fluorescent response. Then the researchers load the coated nanotubes into a needle-thin capillary tube that can safely be implanted into the body. The capillary keeps the nanotubes from directly touching living cells but still allows glucose to enter.
The Illinois researchers tested their glucose sensor by inserting it into a human tissue sample. Then they illuminated the sample with an infrared laser and verified that the strength of the fluorescence from the buried sensor was directly related to the glucose concentrations in the tissue.
-NSF-
Media Contacts
M. Mitchell Waldrop, NSF, (703) 292-8070, email: mwaldrop@nsf.gov
James E. Kloeppel, University of Illinois, Urbana-Champaign, (217) 244-1073, email: kloeppel@uiuc.edu
Program Contacts
Glenn L. Schrader, NSF, (703) 292-8371, email: gschrade@nsf.gov
Principal Investigators
Michael S. Strano, University of Illinois, Urbana-Champaign, (217) 333-3634, email: strano@uiuc.edu
The U.S. National Science Foundation propels the nation forward by advancing fundamental research in all fields of science and engineering. NSF supports research and people by providing facilities, instruments and funding to support their ingenuity and sustain the U.S. as a global leader in research and innovation. With a fiscal year 2023 budget of $9.5 billion, NSF funds reach all 50 states through grants to nearly 2,000 colleges, universities and institutions. Each year, NSF receives more than 40,000 competitive proposals and makes about 11,000 new awards. Those awards include support for cooperative research with industry, Arctic and Antarctic research and operations, and U.S. participation in international scientific efforts.
Connect with us online
NSF website: nsf.gov
NSF News: nsf.gov/news
For News Media: nsf.gov/news/newsroom
Statistics: nsf.gov/statistics/
Awards database: nsf.gov/awardsearch/
Follow us on social
Twitter: twitter.com/NSF
Facebook: facebook.com/US.NSF
Instagram: instagram.com/nsfgov


