All Images
News Release 06-067
Crystal Sieves, Born Anew
Hard data resolves decades-old mystery of how certain zeolites form
This material is available primarily for archival purposes. Telephone numbers or other contact information may be out of date; please see current contact information at media contacts.
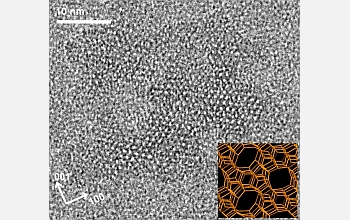
Silicon-oxygen nanoparticles aggregate to form zeolites, capturing other atoms and molecules in the process. The resulting minerals have regularly-shaped, intricate pore and channel systems throughout their structures.
Credit: Michael Tsapatsis, University of Minnesota
Download the high-resolution JPG version of the image. (294 KB)
Use your mouse to right-click (Mac users may need to Ctrl-click) the link above and choose the option that will save the file or target to your computer.
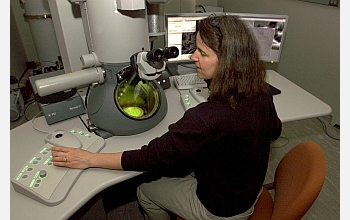
R. Lee Penn of the University of Minnesota department of chemistry uses the Tecnai F30 high-resolution microscope to study zeolite formation. The scope was purchased with the support of a National Science Foundation Major Research Instrumentation grant.
Credit: Patrick O'Leary, University of Minnesota
Download the high-resolution JPG version of the image. (4.9 MB)
Use your mouse to right-click (Mac users may need to Ctrl-click) the link above and choose the option that will save the file or target to your computer.

Tracy Davis, lead author on the zeolite study, used the Small Angle X-Ray Scattering System on loan from Anton Paar GmbH of Granz, Austria, to study zeolite formation and growth.
Credit: University of Minnesota
Download the high-resolution JPG version of the image. (118 KB)
Use your mouse to right-click (Mac users may need to Ctrl-click) the link above and choose the option that will save the file or target to your computer.

Laboratory-grown zeolites appear to form in a hierarchical, steep-by-step fashion with silicon-oxygen nanoparticles forming first. Those particles then aggregate into larger, more complex structures, incorporating other atoms and molecules while still leaving substantial pores and tunnels.
Credit: Michael Tsapatsis, University of Minnesota
Download the high-resolution JPG version of the image. (220 KB)
Use your mouse to right-click (Mac users may need to Ctrl-click) the link above and choose the option that will save the file or target to your computer.

Michael Tsapatis is the principal investigator for the zeolite growth study.
Credit: University of Minnesota
Download the high-resolution JPG version of the image. (238 KB)
Use your mouse to right-click (Mac users may need to Ctrl-click) the link above and choose the option that will save the file or target to your computer.


