From structure to function: Unpacking how genome organization shapes cell behavior
New imaging techniques reveal that the way DNA is packaged enables it to store information and control cellular behavior, much like a computer.
Every cell in the human body contains about 2 meters of DNA, roughly the length of a bed. Though too small to see with the naked eye, DNA is intricately organized, allowing it to govern cellular functions and respond effectively to new information. A study supported by the U.S. National Science Foundation and published in January 2025 shows that the way cells pack their DNA is more flexible than once thought, dynamically acting to form "cellular memories."
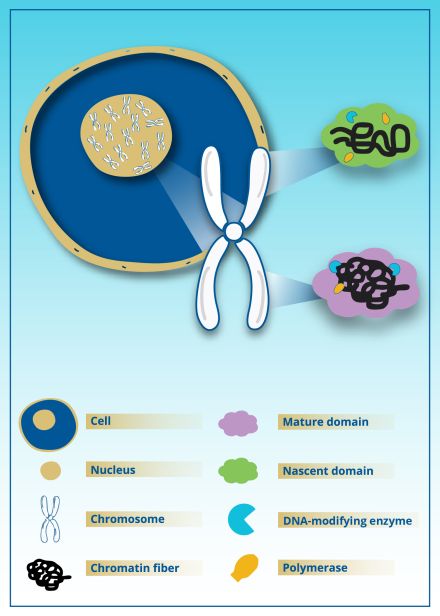
The study, led by Northwestern University biomedical engineer Vadim Backman, revealed a new level of structural sophistication in the folding and organization of chromatin, the DNA-protein complex that comprises chromosomes. Specifically, the researchers found that chromatin packing domains — tiny 3D structures that emerge during this folding process — are far more dynamic than once believed. Rather than being fixed in either an "on" or "off" state, these structures learn and then guide which genes are active and to what level they are expressed. Researchers believe cells may "remember" certain patterns in this structure, helping them respond in predictable ways to changes in their environment.
This discovery sheds new light on the role of chromatin, particularly how its structure reflects gene activity, and offers insights that could have wide-reaching impacts by enabling earlier detection and potentially allowing researchers to rewrite cellular memory errors that contribute to aging and disease.
How DNA computes
In their paper "Chromatin conformation, gene transcription and nucleosome remodeling as an emergent system," Backman and his team used new imaging technologies capable of viewing chromatin at a level of detail never seen before, along with computer modeling and other tools to study how it is packed inside cells. Traditionally, it was believed that chromatin exists in two states: tightly packed regions to keep genes switched off and loosely packed regions to switch genes on.
However, this new research shows it's not that simple. Instead, tiny subregions called nanoscale packing domains pair tight and loose configurations together. Loosely packed configurations at the top of these structures guide the cell's machinery to access and read DNA — a process that leads to RNA and, eventually, proteins. Tightly packed configurations are found deeper in the structure, acting as scaffolding to provide stability. Over time, these subregions can repack themselves more tightly or loosely, adjusting which genes are expressed.
"This finding suggests that cells can remember and reuse patterns in how their chromatin is packed — helping them adapt as their needs change," said Diana Chu, an NSF Directorate for Biological Sciences program officer. "It's like a toolbox that can be rearranged for different tasks. Over time, you learn which configurations work best for specific jobs, allowing you to reuse them when needed."
Unlocking new possibilities for health and aging
This structure-function relationship helps answer longstanding biological puzzles. For example, previous research has found that turning off certain enzymes that usually keep genes quiet, which had been expected to make those genes more active, can surprisingly lead to less gene activity. The reason? These "off" enzymes support the scaffold of chromatin packing domains. When that support is removed, the DNA's built-in memory system becomes disrupted, and the cell struggles to read the genetic instructions correctly.
This discovery gives scientists and engineers a new way to understand — and possibly influence — how cells work. One powerful application lies in health and disease: Small changes in the structure of chromatin might act as early warning signs that a cell is starting to become unhealthy, even before any symptoms appear. This could lead to earlier detection of diseases like cancer and more effective ways to catch and treat problems before they develop.
The research also offers insight into aging. Over time, the system that helps cells "remember" what they are and how they should function can break down. As the chromatin scaffolds become less stable, cells may lose their ability to perform their proper functions. Understanding how this memory fades could eventually lead to ways to slow down or even reverse some of the effects of aging.
Another exciting possibility is the ability to reprogram cells. If scientists and engineers learn how to reshape chromatin’s structure, they could guide cells to take on new roles, like healing damaged tissue, strengthening the immune system or correcting gene activity before problems arise. “The power of this approach is that it spans across human health - all human cells use this processing system. The genome of every human cell continuously learns new cell memories like how we program AI systems,” said Dr. Luay Almassalha, a physician-scientist in gastroenterology and lead author of the study. “What’s remarkable is that cell memory functions as a self-learning system, continually updating itself based on new information and environmental cues. It’s akin to how we design AI algorithms to adapt and improve over time.”
Building the theory behind cellular memory
This discovery builds on nearly 20 years of research by Backman and his team showing that chromatin is more than just structural support — it actively helps control how cells behave. Using advanced imaging and computer modeling, they developed techniques to see how DNA folds inside cells and how that folding affects which genes are turned on.
“NSF started supporting this research at a time when the idea that genome structure may play a critical role in disease ran against the prevailing opinion and required converging biological, physical and computational sciences,” Backman said. “It took a lot of engineering to develop technologies to enable these discoveries. NSF took on an early idea, and now advancements in science and engineering are opening new possibilities for treating Alzheimer's disease, cancer and even reversing aging. Revolutionary developments take time, and NSF's role has been crucial in fostering this emerging field of science and engineering.”
Additional research was conducted at the BioCryo Facility, part of the NSF-funded Soft Hybrid Nanotechnology Experimental (SHyNE) Resource and the Northwestern University Materials Research Science and Engineering Center. Access to these cutting-edge nanotechnology tools helped bring together expertise from biology, physics and engineering. This research was made possible in part by long-term support from NSF, which has backed Backman’s pioneering efforts for close to two decades.
"NSF’s support for Professor Backman’s research, from his early exploratory ideas within core programs through team research programs to center programs, reflects the commitment of NSF and its staff to funding bold ideas that bridge disciplines and transform science and society," said Sohi Rastegar, head of the NSF Office of Emerging Frontiers and Multidisciplinary Activities. "This work showcases how long-term funding can lead to new insights into life at the molecular level."